The RapidFor™ Saliva Antigen Test Kit uses colloidal label as a tool for SARS-CoV-2 antigen detection in the collected saliva specimen.
Advantages
- Easy to use
- Pain-free, no swab
- No extra lab equipment
- Variant Detection [B.1.1.7 (Alpha), B.1.351 (Beta), E484K mutation, B1.617.2 (Delta), P.1 (Gamma), C.37 (Lambda), B.1.1.529 (Omicron)]
- Early phase detection
- Suitable for kids
- Sensitivity 95.3%
- Specificity 99.1%
- Fast Result 15 Mins.
Explanation
RapidFor™ Saliva Antigen Test Kit (Apparatus) is used for in vitro qualitative antigen-detection from SARS-CoV-2 the virus causes COVID-19.
It is intended for the diagnosis of SARS-CoV-2 infection in human saliva specimens from individuals who meet COVID-19 clinical criteria.
RapidFor™ Saliva Antigen Test Kit (Apparatus)allows for the early detection of SARS-CoV-2 in human saliva samples, which causes that people can be quarantined before developing symptoms if SARS-CoV-2 is diagnosed early.
With RapidFor™ Saliva Antigen Test Kit (Apparatus), the asymptomatic infections can be detected and the spread of the COVID-19 pandemic can be controlled.

25 Pcs. Product
Easy to Use
Just collect the sample and test it.
Fast Results
Very simple, and quick results within the next 15 minutes.
High Accuracy
Diagnostic accuracy of the rapid tests are higher for suspected patients (97.4%).
Mass-Scale Testing
Rapid tests can be used in large-scale.
5Pcs. Product
Reliable Products
Our tests are approved and certified by authorities (CE, IVD, ISO, BfArM).
Variant Detection
The results demonstrate that most SARS-CoV-2 (COVID-19) mutated virus strains, including B.1.1.7 (Alpha), B.1.351 (Beta), E484K mutation, B1.617.2 (Delta), P.1 (Gamma), C.37 (Lambda), B.1.1.529 (Omicron) are detectable.
Pain Free
Rapid test provides quick and pain-free test for worried users.

How To Use
RapidFor™ SARS-CoV-2 Saliva Antigen Test Kit (Apparatus) is designed to run in vitro antigen-detection from SARS-CoV-2 qualitatively. It is intended for the diagnosis of SARS-CoV-2 infection in human saliva specimens from individuals who meet COVID-19 clinical criteria.
STEP 1
1. Open the collection tube which is filled with extraction buffer.
STEP 2
2. Carefully place the collecting apparatus to the mouth of the tube.
STEP 3
3. Put your saliva sample into the collection tube with the help of apparatus.
STEP 4
4. Close the buffer tube.
STEP 5
5. Gently flip and mix the liquid.
STEP 6
6. Break off the tip of the cap at the selected point.
STEP 7
7. Add 3 drops of sample-extraction buffer mixture to the sample well and read results after 15 mins.
Interpretation of the Result
This product can only perform qualitative analysis on the detection object.
Positive Result: If both C and T lines are visible within 15 minutes, the test result is positive and valid.
Negative Result: If test area (T line) has no color and the control area displays a colored line, the result is negative and valid.
Invalid Result: The test result is invalid if a colored line does not form in the control region. The sample must be re-tested, using a new test cassette.
*Foot-note1: The samples should be used as soon as possible after collected (within half an hour). Samples should not be inactivated.
*Foot-note2: Recommend using a pipette to transfer the samples to reduce deviations
*Foot-note3: Dispose of all the materials used during the test to biological waste especially the result of the test is positive.
Usage Areas
Each of us should play a role in reducing the danger of the COVID-19 spreading and resuming economic and social activities in a safe environment.
Anyone who desires to attend a place where certain events, businesses, or activities are being organized must perform the COVID-19 test.
The test provides a qualitative result showing colored bands indicating the presence of COVID-19 (SARS CoV-2) antigens. Saliva Antigen Test Kit (Apparatus) is used for screening purposes in many places such as hospitals, clinics, laboratories, airports, hotels, schools, restaurants, and production facilities.
To attend an event, test results must be provided within 72 hours. It is everyone's responsibility to stop the COVID-19 from spreading.








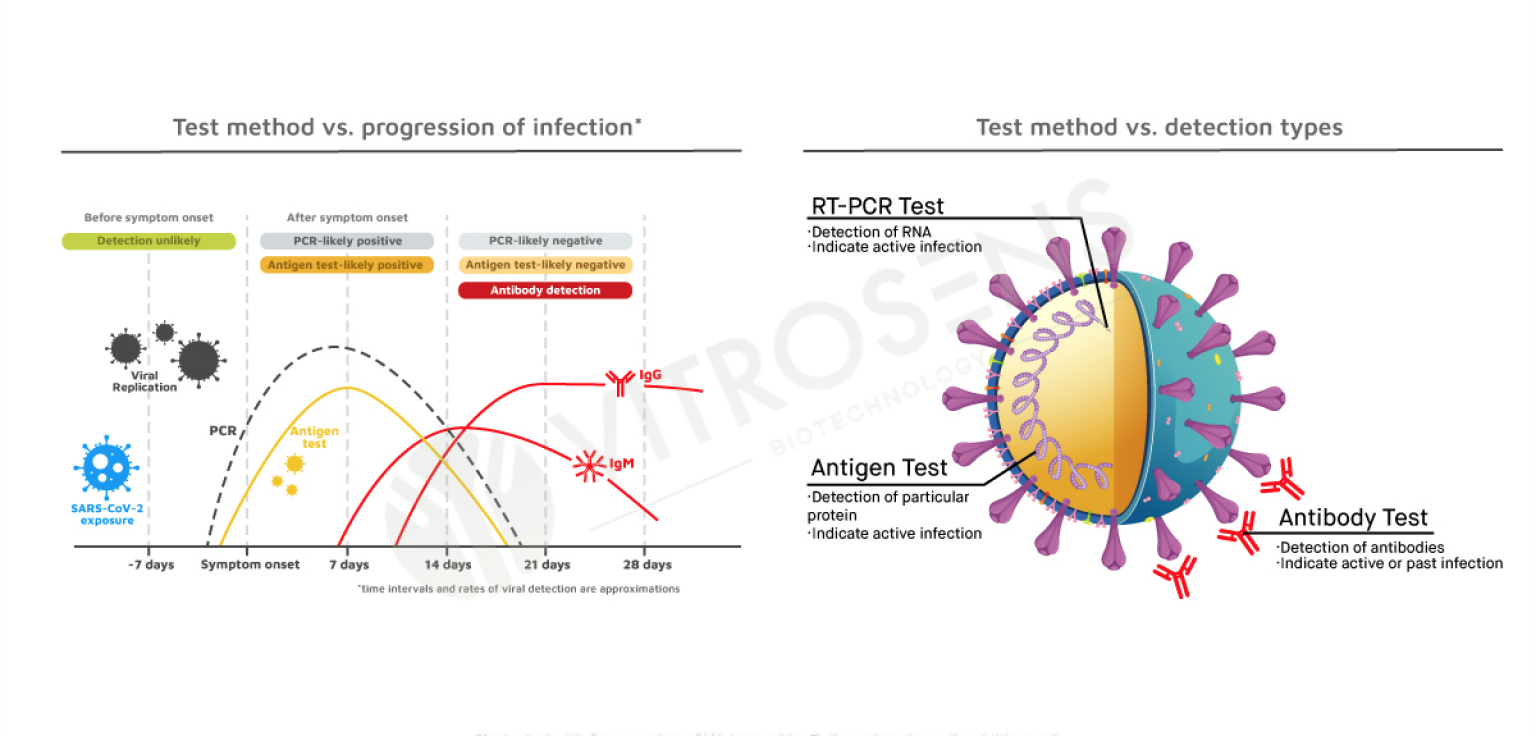
Test Types Comparison
| Test Types | PCR | Antigen | ELISA | Antibody |
|---|---|---|---|---|
| Detected | Nucleic Acid | Antigen | Antibody | Antibody |
| Sample Type | Nasopharyngeal Oropharyngeal Swab | Nasal Nasopharyngeal Oropharyngeal Saliva Swab | Serum and Plasma | Serum Plasma and Whole Blood |
| Time To Result | 3 Hours | 15 Minutes | 45 Minutes | 15 Minutes |
| Device Requirement | Yes | No | Yes | No |
| Lab Requirement | Yes | No | Yes | No |
Box Content

| Content | 25 Test Option | 5 Test Option |
|---|---|---|
| Test Casette | 25 Pcs. | 5 Pc. |
| Extraction buffer | 25 Pcs. | 5 Pc. |
| Collecting Apparatus | 25 Pcs. | 5 Pc. |
| Procedure Card (Optional) | 1 Pc. | 1 Pc. |
| User Manual | 1 Pc. | 1 Pc. |
Order Information
| PRODUCT | CATALOG NO | PACK SIZE | Pcs/Carton | Pcs/ Euro Palette | Carton Size | Euro Palette Size |
|---|---|---|---|---|---|---|
| RapidFor™ Saliva Antigen Test Kit (Apparatus) | VSCD05-5 | 1 Box / 5 Pc. | 500 | 6000 | 71x54x36 | 80×120 |
| RapidFor™ Saliva Antigen Test Kit (Apparatus) | VSCD05-25 | 1 Box / 25 Pcs. | 1000 | 20000 | 58x45x43 | 100×120 |
Frequently Asked Questions
RapidFor™ Saliva Antigen Test Kit (Apparatus) has 97.4 percent accuracy according to clinical studies. Kindly refer to the “Instructions for Use (IFU)” for more details.
Yes, the test is able to detect the virus at an early stage (2-4 days after viral exposure). Please see the “Test Types Comparison” part for further information.
COVID-19 viral variations are being actively monitored by the RapidFor™ Saliva Antigen Test Kit (Apparatus) to verify that our assays can detect them, as we do with many viruses. We have made a complete analysis of the existing variants including B.1.1.7 UK (Alpha), B.1.351 South Africa (Beta), E484K mutation, B1.617.2 India (Delta), P.1 Brazil (Gamma), C.37 (Lambda), B.1.1.529 (Omicron); we have seen, and we’re certain that our tests will still detect these strains. The test looks for proteins that the COVID-19 virus needs to live.
In comparison with the Real-Time Polymerase Chain Reaction Test (RT- PCR Test), RapidFor™ Saliva Antigen Test Kit enables us to screen the existence of the illness of concern in a very short time. This benefit gives us the opportunity to test more people in less time. It is a major advantage in the event of worldwide pandemics such as COVID-19. Since many of the COVID-19 symptoms are similar to those associated with common cold, or other diseases, the testing of a person having or not COVID-19 is required.
Yes, RapidFor™ Saliva Antigen Test Kit (Apparatus) Test Kit can be used in schools because we simplified the procedure: no swab just spit. Due to RapidFor™ SARS-CoV-2 Saliva Antigen Test Kit (Apparatus)requires no swab even children can operate the test by themselves of course under surveillance of an adult.
You should not eat or drink (including water) before collecting saliva sample in the prior 30 minutes. Be careful not to brush or floss your teeth or use mouthwash. You should also avoid chewing gum, lozenges, cough drops, lollipops, suckers, etc. Please make sure not to use any tobacco products.
Before performing the test, please carefully read instruction manual. The process should be completed within 15 minutes of withdrawing the test cassette from the foil package. The buffer solution should be recapped as soon as possible after use. After collecting the samples, they should be used as quickly as feasible (within half an hour). Samples should not be inactivated.
Make contact with your healthcare professional and let them know about your condition. You should get polymerase chain reaction (PCR Test) to confirm the presence of COVID-19 infection. If the illness is confirmed, you should isolate yourself and follow your local public health regulations. Inform your coworkers and others you met in two weeks during your self-isolation.
Our tests are quite accurate, but you can never be completely certain. Because the viral load in your body may not be substantial enough to be detected by the test, you should repeat the test after 4-5 days. Remember to keep a safe distance from others and stay away from potentially dangerous situations.
Absolutely no, the test cassette is designed to be used just once. It is strongly advised against testing another sample on the same cassette.
Put everything you used for the process in the aluminum foil, including the swab, tube, collecting apparatus and test cassette. Do not put them in the recycling bin; instead, throw them away. Animals and children should be kept out of reach. If you have access to one, dispose of all of the items used during the test in medical waste.
Store in the sealed bag between 2°C and 30°C until the expiration date on the packaging is reached; do not store below 2°C or consume expired products. On the label, the manufacture and expiration dates are printed. The product will no longer be used after 24 months.