The RapidFor™ Saliva Antigen Test Kit (Swab) uses colloidal label as a tool for SARS-CoV-2 antigen detection in the collected saliva swab specimens.
Advantages
- Easy to use
- Pain-free
- No extra lab equipment
- Variant Detection [B.1.1.7 (Alpha), B.1.351 (Beta), E484K mutation, B1.617.2 (Delta), P.1 (Gamma), C.37 (Lambda), B.1.1.529 (Omicron) ]
- Early phase detection
- Easy to store and transport
- Sensitivity 97.03%
- Specificity 99.22%
- Fast Result 15 Mins.
Explanation
RapidFor™ Saliva Antigen Test Kit (Swab)is created for the screening of SARS-CoV-2 infection in human saliva swab specimens from suspected COVID-19 patients. SARS-CoV-2 antigen-detecting is a cheaper, faster method to detect acute phase of COVID-19.
RapidFor™ Saliva Antigen Test Kit (Swab) offers an easy way to detect SARS-CoV-2 in early stage from human saliva swab samples. With the early diagnosis of SARS-CoV-2, people can be isolated before they develop symptoms.
Currently, the people tainted by SARS-CoV-2 are the main source of COVID-19 spread around the world. With RapidFor™ Saliva Antigen Test Kit (Swab), the asymptomatic infections can be detected and the spread of the COVID-19 pandemic can be controlled.

25 Pcs. Product
Easy to Use
Just collect the sample and test it.
Fast Results
Very simple, and quick results within the next 15 minutes.
High Accuracy
Diagnostic accuracy of the rapid tests are higher for suspected patients (98.25%).
Mass-Scale Screening
Rapid tests can be used in large-scale.
5Pcs. Product
Reliable Products
Our tests are approved and certified by authorities (CE, IVD, ISO, BfArM).
Variant Detection
The results demonstrate that most SARS-CoV-2 (COVID-19) mutated virus strains, including B.1.1.7 (Alpha), B.1.351 (Beta), E484K mutation, B1.617.2 (Delta), P.1 (Gamma), C.37 (Lambda) and B.1.1.529 (Omicron) are detectable.
Pain Free
Rapid test provides quick and pain-free test for worried users

How To Use
Saliva Antigen Test Kit (Swab)
Rapidfor™ Saliva Antigen Test Kit (Swab) is designed to run the in vitro antigen-detection from SARS-CoV-2, qualitatively. It is intended for the diagnosis of SARS-CoV-2 infection in saliva swab specimens from individuals who meet COVID-19 clinical criteria.
STEP 1
1. Place the swab under the tongue for 10 seconds, rotate 5 times and soak it completely.
STEP 2
2. Open the extraction buffer tube.
STEP 3
3. Put your sample collection swap into the tube.
STEP 4
4. Rotate the swab 10 times to elute the sample into the buffer.
STEP 5
5. Break off the upper part of the swab with squeezing.
STEP 6
6. Close the buffer tube.
STEP 7
7. Gently flip and mix the liquid.
STEP 8
8. Break off the tip of the cap at the selected point.
STEP 9
9. Add 3 drops of sample-extraction buffer mixture to the sample well and read results after 15 mins.
Interpretation of the Result
Saliva Antigen Test Kit (Swab)
This product can only perform qualitative analysis on the detection object.
Positive Result: If both C and T lines are visible within 15 minutes, the test result is positive and valid.
Negative Result: If test area (T line) has no color and the control area displays a colored line, the result is negative and valid.
Invalid Result: The test result is invalid if a colored line does not form in the control region. The sample must be re-tested, using a new test cassette.
*Foot-note1: The samples should be used as soon as possible after collected (within half an hour). Samples should not be inactivated.
*Foot-note2: Recommend using a pipette to transfer the samples to reduce deviations
*Foot-note3: Dispose of all the materials used during the test to biological waste especially the result of the test is positive.
How To Use
Saliva Antigen Test Kit (Swab)
Each of us should play a role in reducing the danger of the COVID-19 spreading and resuming economic and social activities in a safe environment.
Anyone who desires to attend a place where certain events, businesses, or activities are being organized must perform the COVID-19 test.
The test provides a qualitative result showing colored bands indicating the presence of COVID-19 (SARS CoV-2) antigens. Rapid Antigen Test Kit (Self-Test) is used for screening purposes in many places such as hospitals, clinics, laboratories, airports, hotels, restaurants, and production facilities.
To attend an event, test results must be provided within 72 hours. It is everyone's responsibility to stop the COVID-19from spreading.








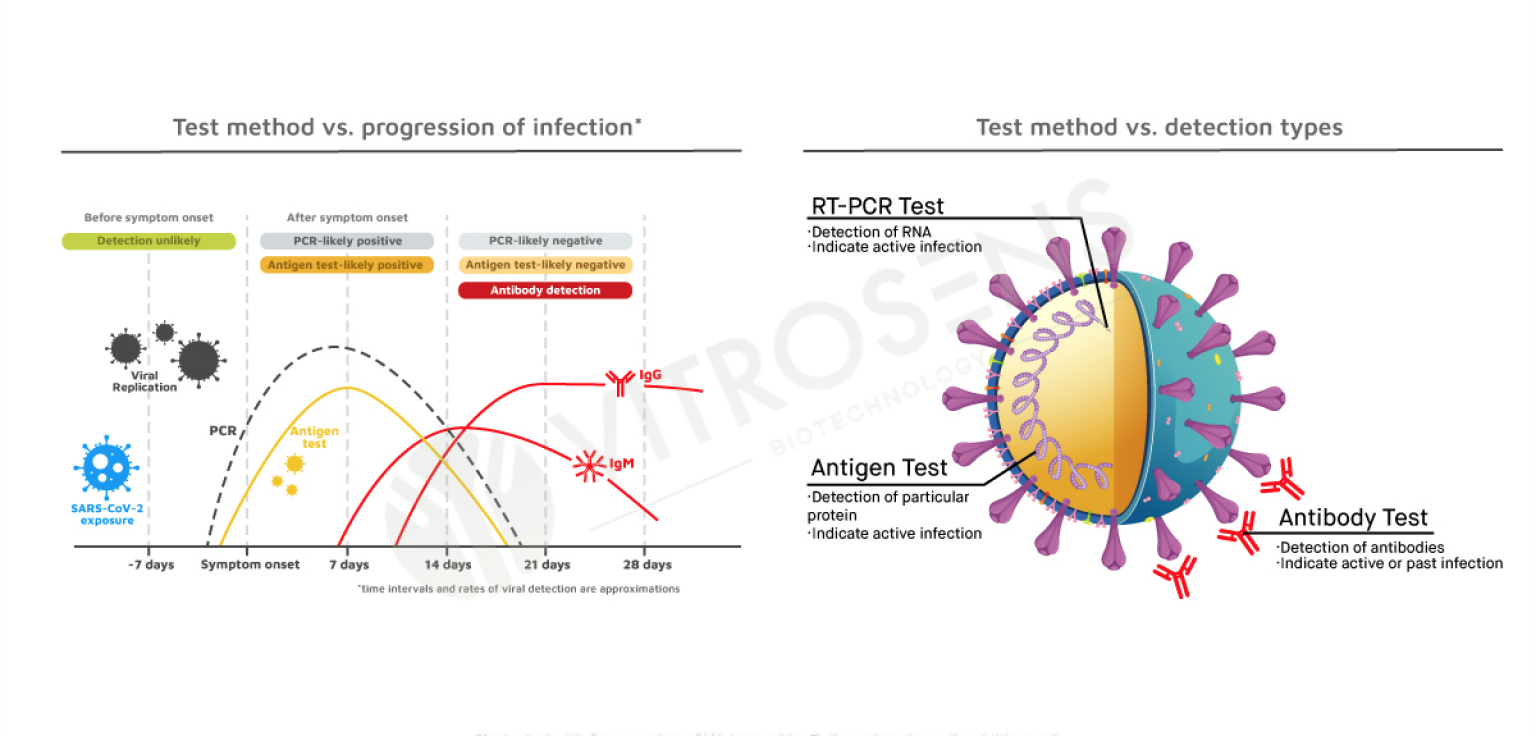
Test Types Comparison
Rapid Antigen Test Kit (Self-Test)
| Test Types | PCR | Antigen | ELISA | Antibody |
|---|---|---|---|---|
| Detected | Nucleic Acid | Antigen | Antibody | Antibody |
| Sample Type | Nasopharyngeal Oropharyngeal Swab | Nasal Nasopharyngeal Oropharyngeal Saliva Swab | Serum and Plasma | Serum Plasma and Whole Blood |
| Time To Result | 3 Hours | 15 Minutes | 45 Minutes | 15 Minutes |
| Device Requirement | Yes | No | Yes | No |
| Lab Requirement | Yes | No | Yes | No |
Box Content
Saliva Antigen Test Kit (Swab)

| Content | 5 Test Option | 2 Test Option | Single Test Option |
|---|---|---|---|
| Test Casette | 5 Pcs. | 2 Pcs. | 1 Pc. |
| Nasal Swab | 5 Pcs. | 2 Pcs. | 1 Pc. |
| Extraction Buffer | 5 Pcs. | 2 Pcs. | 1 Pc. |
| Biohazard Bag (Optional) | 5 Pcs. | 2 Pcs. | 1 Pc. |
| User Manual | 1 Pc. | 1 Pc. | 1 Pc. |
Order Information
Saliva Antigen Test Kit (Swab)
| PRODUCT | CATALOG NO | PACK SIZE | Pcs/Carton | Pcs/ Euro Palette | Carton Size | Euro Palette Size |
|---|---|---|---|---|---|---|
| RapidFor™ Saliva Antigen Test Kit (Swab) | VSCD05-5 | 1 Box / 5 Pc. | 500 | 6000 | 71x54x36 | 80×120 |
| RapidFor™ Saliva Antigen Test Kit (Swab) | VSCD05-25 | 1 Box / 25 Pcs. | 1000 | 20000 | 58x45x43 | 100×120 |
Frequently Asked Questions
Saliva Antigen Test Kit (Swab)
Clinical studies show that the RapidFor™ Saliva Antigen Test Kit (Swab) is 97.4 percent accurate. For additional information, please read the “Instructions for Use (IFU)”.
Yes, the test is capable of measuring the virus early stage (2-4 days after viral exposure). For further details, check the “Test Types Comparison” section.
The RapidFor™ Saliva Antigen Test Kit (Swab) is constantly monitoring COVID-19 viral variants to ensure that our tests can identify them, as we do with many viruses. We have performed a proper examination of the current variations including B.1.1.7 UK (Alpha), B.1.351 South Africa (Beta), E484K mutation, B1.617.2 India (Delta), P.1 Brazil (Gamma), C.37 (Lambda), B.1.1.529; and we are certain that our tests will still detect these strains. The test looks for proteins that are required for the COVID-19 virus to survive.
In comparison with the Real-Time Polymerase Chain Reaction Test (RT- PCR Test), RapidFor™ SARS-CoV-2 Saliva Antigen Test Kit (Swab) enables us to screen the existence of the illness of concern in a very short time. This benefit gives us the opportunity to test more people in less time. It is a major advantage in the event of worldwide pandemics such as COVID-19. Since many of the COVID-19 symptoms are similar to those associated with common cold, or other diseases, the testing of a person having or not COVID-19 is required.
When the end of the oropharyngeal swab is placed in your mouth, it should not hurt. If you are experiencing any pain, stop the test and seek medical help.
Before taking saliva swab samples, you should not eat or drink anything (even water) in the prior 30 minutes. Avoid using mouthwash or brushing or flossing your teeth. Chewing gum, lozenges, cough drops, lollipops, suckers, and other similar items should also be avoided. Please refrain from using any tobacco products.
Kindly read the instruction booklet attentively before doing the test. Within 15 minutes of removing the test cassette from the foil package, the procedure should be finished. After each usage, the buffer solution should be recapped as quickly as feasible. The samples should be utilized as soon as possible after being collected (within half an hour). Inactivation of samples is not recommended.
Make touch with your healthcare provider and inform them of your situation. To confirm the presence of COVID-19 infection, utilize polymerase chain reaction (PCR Test). If you have been diagnosed with a disease, you should isolate yourself and obey your local public health rules. Inform your employees and others you met throughout your two-week self-isolation period.
Our tests are quite accurate, but there is no way to know for sure. You should repeat the test after 4-5 days since the viral load in your body may not be significant enough to be identified by the test. Keep a healthy distance between yourself and others and stay away from potentially harmful situations.
No, the test cassette is only meant to be used once. It’s not a good idea to test another sample on the same cassette.
Put everything you used for the process, including the swab, tube, and test cassette, in the aluminum foil. Instead of putting them in the recycling container, throw them away. Children and animals should be kept apart from one other. If you have one, put all of the materials used during the test in a medical waste container.
Store between 2°C and 30°C in the sealed bag until the expiration date on the packaging is reached; do not store below 2°C or consume expired items. The manufacturing and expiration dates are printed on the label. After 24 months, the product will no longer be used.